(C) 2013 Maj-Britt Pontoppidan. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Pontoppidan M-B, Nachman G (2013) Changes in behavioural responses to infrastructure affect local and regional connectivity – a simulation study on pond breeding amphibians. Nature Conservation 5: 13–28. doi: 10.3897/natureconservation.5.4611
An extensive and expanding infrastructural network destroys and fragments natural habitat and has detrimental effect on abundance and population viability of many amphibian species. Roads function as barriers in the landscape. They separate local populations from each other or prevent access to necessary resources. Therefore, road density and traffic intensity in a region may have severe impact on regional as well as local connectivity. Amphibians may be able to detect and avoid unsuitable habitat. Individuals’ ability to avoid roads can reduce road mortality but at the same time road avoidance behaviour, can increase the barrier effect of the road and reduce connectivity. We use an individual based model to explore how changes in road mortality and road avoidance behaviour affect local and regional connectivity in a population of Moor frogs (Rana arvalis). The results indicate that road mortality has a strong negative effect on regional connectivity, but only a small effect on local connectivity. Regional connectivity is positively affected by road avoidance and the effect becomes more pronounced as road mortality decreases. Road avoidance also has a positive effect on local connectivity. When road avoidance is total and the road functions as a 100% barrier regional connectivity is close to zero, while local connectivity exhibit very elevated values. The results suggest that roads may affect not only regional or metapopulation dynamics but also have a direct effect on local population dynamics.
Rana arvalis, Individual based modelling, Road avoidance, Road mortality, Connectivity, Pond breeding amphibians
All over the world, amphibian populations are declining and many amphibian species are listed in the IUCN as threatened or vulnerable (
Very little literature exists on amphibians’ reactions to roads. The only study on this topic did not find any indication of road avoidance in Rana pipiens (
Pond breeding amphibians require both terrestrial and aquatic habitat to complete their life cycle. Proximity between the required habitat types is important for the survival of the population. Loss of, or diminished access to, one or both habitats will affect population size and persistence probability (
We have developed an individual based model to assess the effects of infrastructure on landscape connectivity. The model is part of a larger study concerning road effects on regional populations of Moor frogs (Rana arvalis). In this paper we present our model and explore how behavioural responses to infrastructure may affect local and regional connectivity. The ability to avoid roads may diminish the amount of road kills. This behaviour will prevent dispersal across the road but at the same time it may affect connectivity locally. Lower levels of road avoidance can reduce the road’s barrier effect but this will probably depend on the level of road mortality. We hypothesize that
- Regional connectivity will be inhibited by high levels of road avoidance and high road mortality and will depend on interactions between the degree of road avoidance and road mortality.
- Local connectivity will be promoted by high levels of road avoidance but not be affected by road mortality.
We use a real Danish landscape with a population of Moor frogs (Rana arvalis) traversed by a large road to test how regional and local connectivity are affected by changes in road mortality and road avoidance.
We use an individual based model to simulate the movements of juvenile Moor frogs and estimate immigration probabilities between habitat patches. The purpose of the model is to measure the connectivity of the landscape. In the following we use the terms dispersal and migration as defined by
Moor frogs spend most of their life in terrestrial habitat; aquatic habitat is only used during the breeding season, which takes place in the early spring (
Full model documentation following the ODD-template suggested by
The model considers a regional population of Moor frogs within a spatially explicit landscape matrix. The landscape is constructed from a 600 × 800 cell GIS raster map, each cell representing an area of 10 × 10 meters. A raster cell is characterised by a set of variables defining the habitat type and its value in regard to the different aspects of the life cycle and behaviour of the Moor frog (Table 1). Potential sites for subpopulations of Moor frogs are represented by a GIS point-data set of ponds surveyed during field work. Each pond is defined by an ID-number, a quality index and the summer habitat fragments located within migration distance from the pond (Table 1).
Immigration requires two events: 1) the successful dispersal of a juvenile frog to summer habitat outside its natal habitat patch and 2) subsequent successful migration from the new summer habitat to a nearby breeding pond. In real life dispersal starts just after metamorphosis in early summer and lasts until hibernation in the autumn. The second part of the immigration event, migration, takes place in the spring 2.5 years later. For simplicity, we simulate the dispersal and breeding migration, as if they take place in the same year.
List of variables characterizing the agents in the model.
| Variable | Notation | Value range | Agent type | Description |
|---|---|---|---|---|
| DailySurvival | Ds | Cell | Daily survival probability | |
| HabitatAttraction | Ha | 1–5 | Cell | The cell’s relative attraction to frogs during movement |
| HabitatCode | Hc | Cell | Cell code for habitat type | |
| HabitatSurvival | Hs | 1–5 | Cell | The cell’s relative survival index |
| SummerQuality | Hq | 1–5 | Cell | The cell’s relative suitability as summer habitat |
| BreedingPond | Frog | Breeding pond of frog agent | ||
| NatalPond | Frog | Natal pond of frog agent | ||
| PondID | Pond | ID number | ||
| PondQuality | Q | 0.1–1 | Pond | Quality index of the pond |
| SummerHabitat | A | Pond | Summer habitat cells associated with the pond |
The time step of the model is one day and the simulated period for dispersal as well as migration is 120 days each. At the start of a simulation, 500 frog agents are created at each pond. Each agent is assigned a random direction, which determines its preferred direction of movement. This direction does not change unless summer habitat is found. At each time step, a random daily travelling distance is chosen for each agent; the distance depending on the attractiveness of the current habitat. The distance is travelled one cell at a time. Depending on the relative attractiveness of the neighbouring cells, frog agents move to one of the cells, although backwards movement is not allowed. The movement rules generate a biased random walk away from the natal pond and in the preferred direction. During dispersal, frog agents encountering a cell with summer habitat will have a certain probability of settling in the habitat and stop dispersing. This probability will increase with time. At the end of the dispersal period all frog agents that have not settled in summer habitat are removed. Starting the migration phase, the remaining frog agents move toward the breeding pond associated with their summer habitat; in case several breeding ponds are available one is chosen randomly weighted by pond quality. After each time step, the survival probability of every frog agent is assessed, based on the daily survival rates associated with the habitat type traversed during the day.
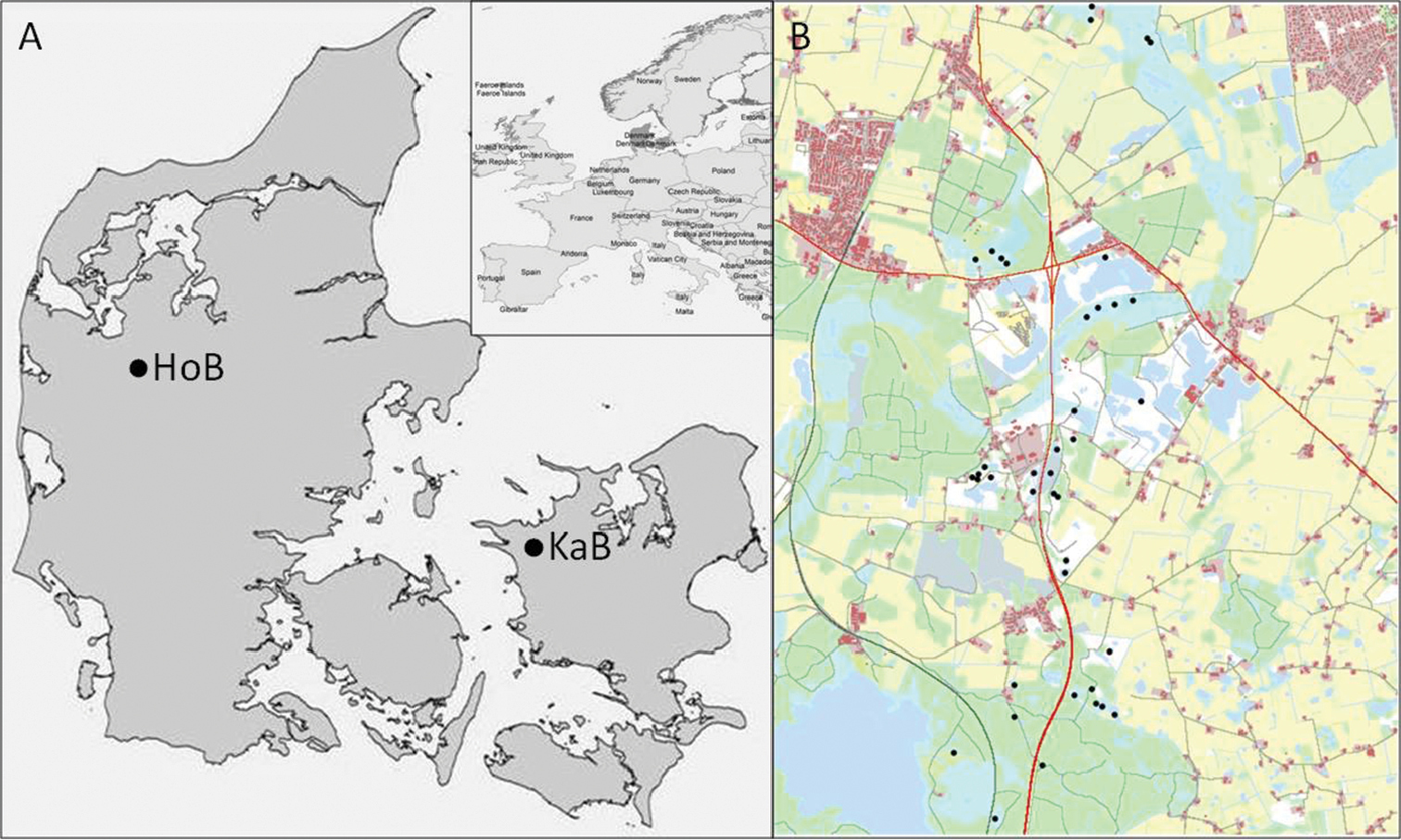
We use GIS data sets from a road project in Denmark, supplied by the Danish Road Directorate and Amphi Consult. The project concerns an area in north-western part of Zealand, 10 km east of the city of Kalundborg (55°40.14'N, 11°17.85'E) (Fig. 1). The area is characterised as semi-urban and agricultural landscapes, traversed by creeks and wetlands. A project data set contains a land cover map of the area and a point-data set of potential breeding ponds found during field surveys. The land cover maps are constructed following a protocol designed by amphibian experts (
Study area. A Location of two study areas in Denmark. KaB is an area near Kalundborg on Zealand and HoB is near Holstebro in Jutland. Only KaB is used in the present analysis, but both areas are used for the parameterisation of the model B KaB map used in the analysis. Black dots are breeding ponds, test roads are marked with red.
The point-data set contains information on the location of the potential breeding pond, its ID-number as well as a quality index (Q). Pond qualities ranges from 0.1 – 1 and relates to the suitability of the pond and the immediate surroundings in regard to egg and larval survival and are estimated by experts during field work. In this paper we have excluded low-quality ponds (Q < 0.6), since they per definition have a low probability of maintaining a population on their own. The extent of the map is 6×8 km and it contains 40 ponds.
We create scenarios with increasing values of road avoidance, Ra = [1; 2; 3; 4; 5], and road mortality, Rd = [0.1; 0.3; 0.5; 0.7; 0.9], of the two major roads cutting through the map (Fig. 1, roads shown in red). We run 25 simulations for every combination of the parameter values of Ra and Rd. As Ra increases the willingness of the frog agents to enter the road will decrease, while the probability of surviving will increase with decreasing values of Rd.
At the end of each simulation, the natal pond and the breeding pond of all frog agents are registered and immigration probability (pij) between all pair-wise ponds is calculated. Landscape connectivity (S) is then found as
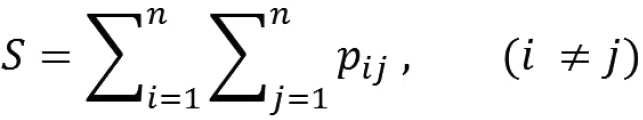 (eq. 1)
(eq. 1)
Local populations are identified by grouping ponds into clusters depending on their mutual connectivity, using the method of unweighted, arithmetic, average clustering as described by
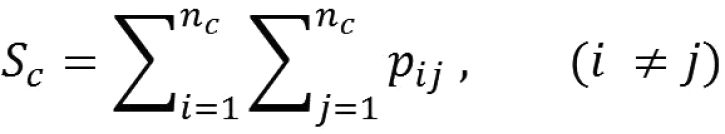 (eq. 2)
(eq. 2)
where nc is the number of ponds belonging to cluster c. Connectivity between clusters (Sb) is then found as Sb = S – Sc. However, to be able to detect changes in local connectivity, the ponds constituting a cluster must be the same in all scenarios. Therefore, we use the cluster configuration found when Ra is set to 5 to define clusters, and use this in all calculations of within-cluster connectivity.
We use a multiple regression model, with the general form y = β0 + β1Rd + β2Ra + β3RdRa + ε, to test for the effect of road avoidance (Ra), road mortality (Rd) and their interaction on landscape connectivity (S), within-cluster connectivity (Sc) and between-cluster connectivity (Sb). Sequential Holm-Bonferroni correction is used to adjust p-values. When Ra is set to 5, frog agents to do not enter the road, therefore the level of road mortality is inconsequential. Moreover, preliminary tests showed extreme connectivity values when the road is 100% blocked. Both of these factors risk masking the statistical effect of road mortality and road avoidance on connectivity at other levels of Ra. Consequently, the results from the scenarios with Ra = 5 are excluded from the statistical testing.
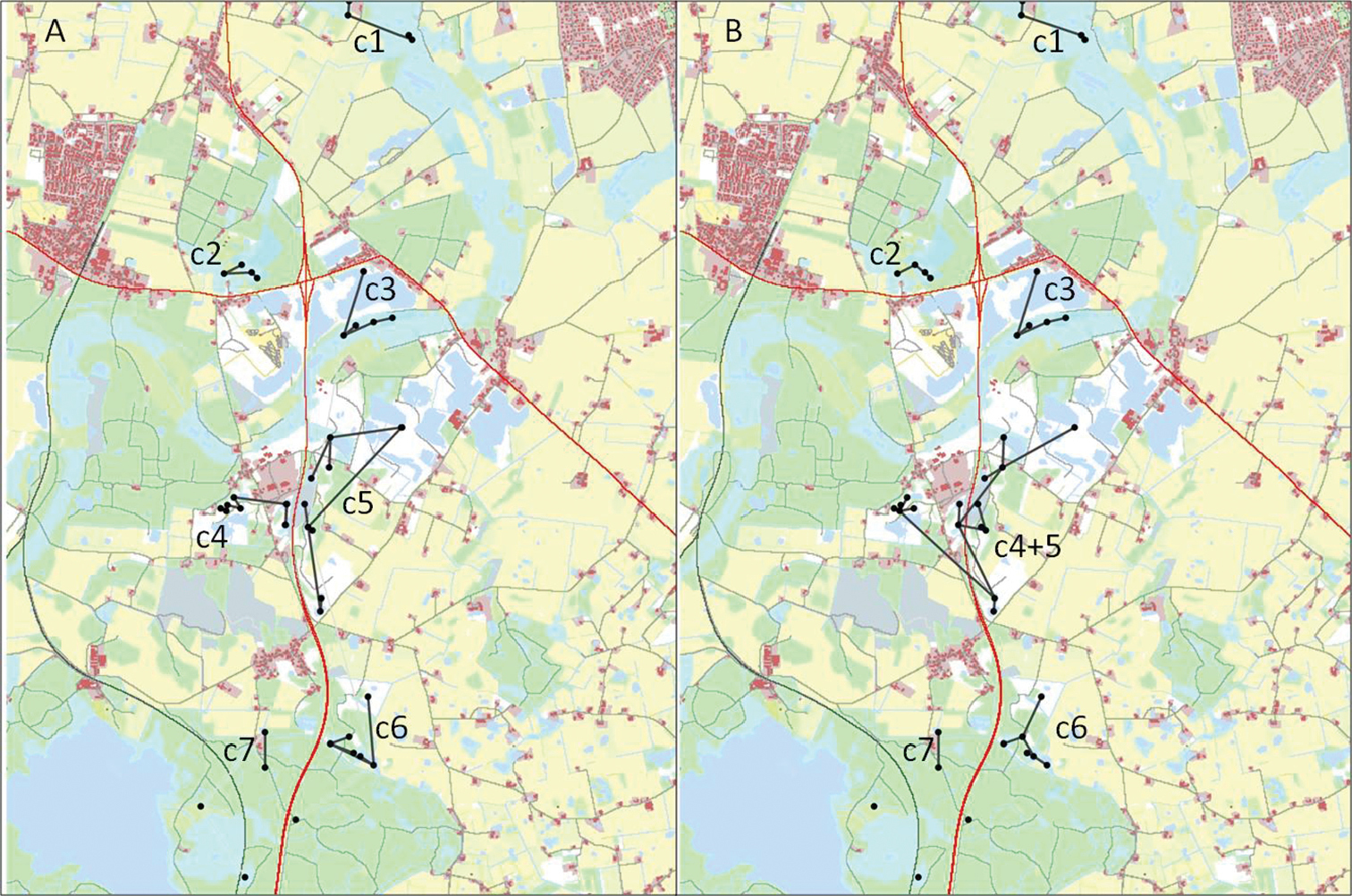
Analyses of the scenarios with Ra = 5 identify seven clusters (Fig. 2A, Table 2). Cluster c1 contains four ponds and is located rather remotely in the top of the map. Clusters c2 and c3 are found in areas close to where the two test roads cross and contains four, respectively, five ponds. Cluster c4 and cluster c5 contains seven and nine ponds, respectively. These are more widespread clusters situated on either side of the road in the middle of the map. The last two clusters c6 and c7 are placed near the bottom of the map and contain two and six ponds. As described in the method section we use this cluster configuration as a reference for all scenarios when calculating within-cluster connectivity. Nonetheless, the analyses show that cluster configurations do not change with the different scenarios except when road mortality is set to 0.1. In this case dispersal success is sufficiently high between cluster c4 and c5 and they fuse into one cluster (Fig. 2B).
Results from cluster analyses with two different parameter settings. A Ra = 5, Rd = 0.1 and B Ra = 4, Rd = 0.1.
Descriptive statistics of the identified clusters. The cluster’s ID, number of ponds in the cluster, mean distance from the ponds to a road, the distance to the pond closest to the road and the number of pond members no more than 200 m from the road. Furthermore it is shown whether the cluster exhibits extreme connectivity values when Ra = 5, its response to road avoidance and its response to road mortality.
| Cluster Id | Cluster characteristics | Respons patterns | |||||
|---|---|---|---|---|---|---|---|
| Cluster size | Mean distance (m) | Min distance (m) | Ponds 200m | Ra = 5 | Avoidance | Mortality | |
| c1 | 4 | 1322 | 1082 | 0 | N | N | N |
| c2 | 4 | 184 | 110 | 3 | Y | Y | N |
| c3 | 5 | 345 | 76 | 1 | Y | Y | N |
| c4 | 7 | 431 | 61 | 2 | Y | Y | Y |
| c5 | 9 | 223 | 71 | 6 | Y | Y | Y |
| c6 | 6 | 323 | 98 | 1 | Y | N | N |
| c7 | 2 | 385 | 318 | 0 | N | N | N |
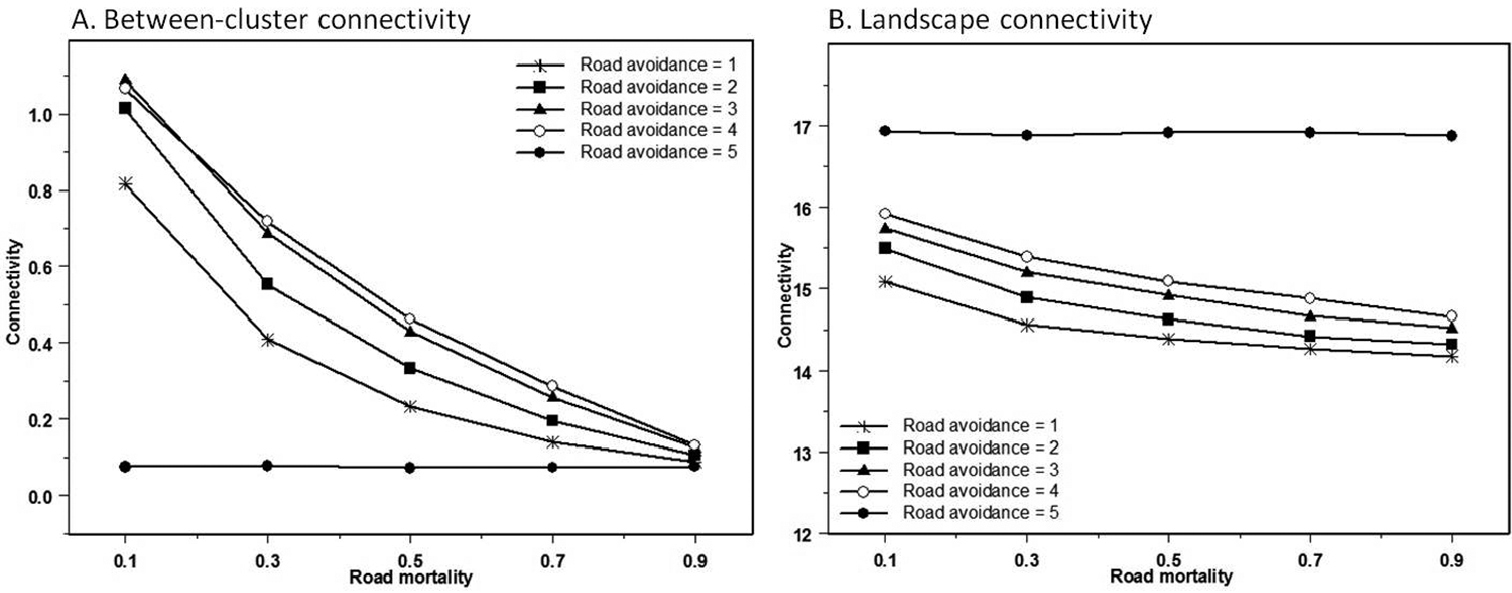
When Ra ≤ 4, road mortality has strong negative effect on the connectivity between clusters (Sb) while road avoidance has a positive effect. Furthermore, there is an interaction effect; the effect of road avoidance becomes more pronounced as road mortality decreases (Table 3). These effects are all statistically significant (F3, 496 = 1814, p <0.001). When Ra = 5, between-cluster connectivity yields its lowest values (Fig. 3A). In these scenarios all dispersal across the road is impossible. Hence, the measured connectivity must represent the connectivity between clusters on the same side of the road.
Effect of road avoidance (Ra) and road mortality (Rd) on A between-cluster connectivity (Sb) and B landscape connectivity (S).
Statistical results of multiple regression models. Statistical significance of variables and interactions in multiple regressions on landscape connectivity (S), within-cluster connectivity (Sc) of cluster c1 – c7 and between-cluster connectivity (Sb). Sequential Holm-Bonferroni correction is used to adjust p-values. Statistically significant values are shown in bold.
| Dependent factor | df | Full model | Road mortality Rd | Road avoidance, Ra | Interaction Ra * Rd | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | R2 | Parameter | p | Parameter | p | Parameter | p | ||
| Sc1 | 496 | 0.30 | 0.82 | 0.002 | -0.003 | 0.873 | 0.001 | 0.738 | 0.001 | 0.884 |
| Sc2 | 496 | 7.20 | <0.001 | 0.042 | 0.018 | 0.281 | 0.012 | <0.001 | -0.014 | 0.018 |
| Sc3 | 496 | 39.31 | <0.001 | 0.192 | -0.024 | 0.240 | 0.021 | <0.001 | -0.020 | 0.007 |
| Sc4 | 496 | 255.1 | <0.001 | 0.607 | -0.053 | 0.014 | 0.064 | <0.001 | -0.012 | 0.121 |
| Sc5 | 496 | 487.9 | <0.001 | 0.747 | -0.094 | <0.001 | 0.092 | <0.001 | -0.006 | 0.522 |
| Sc6 | 496 | 0.85 | 0.45 | 0.005 | -0.014 | 0.496 | 0.002 | 0.739 | 0.002 | 0.775 |
| Sc7 | 496 | 0.81 | 0.49 | 0.005 | -0.005 | 0.584 | 0.001 | 0.676 | 0.0001 | 0.976 |
| Sb | 496 | 1814 | <0.001 | 0.917 | -0.831 | <0.001 | 0.114 | <0.001 | -0.096 | <0.001 |
| S | 496 | 1297 | <0.001 | 0.887 | -1.005 | <0.001 | 0.307 | <0.001 | -0.145 | <0.001 |
Overall landscape connectivity (S) exhibits very elevated values when road avoidance is total. Otherwise, landscape connectivity decreases with road mortality and increases with road avoidance, the effect of road avoidance being strongest at lower values of road mortality (Fig. 3B). All effects are statistically significant (F3, 496 = 1297, p <0.001) (Table 3).
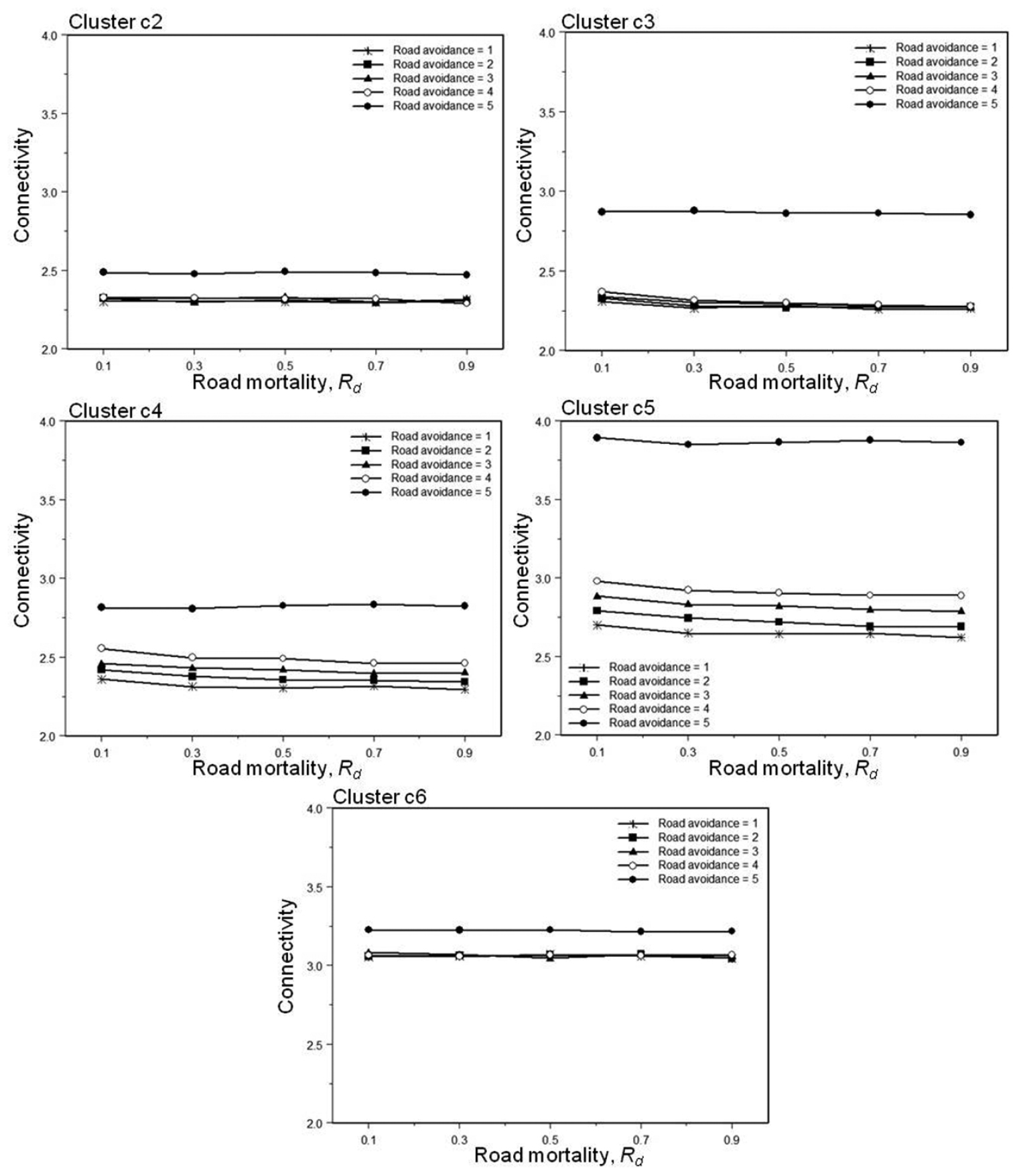
In general, the same trends are found in within-cluster connectivity (Sc) (Table 3). There are, however, some differences. Within-cluster connectivity of cluster c1 and c7 is affected neither by changes in road mortality nor road avoidance. These two clusters are also furthest away from the test roads (Table 2). Clusters c2 trough c6 all exhibit very elevated connectivity values when Ra = 5. Connectivity within these clusters also tend to increase with road avoidance and decrease with road mortality when Ra ≤ 4 (Fig. 4). However, the effect of road mortality is only statistically significant in clusters c4 and c5 (and after Bonferroni correction only c5). Both are large clusters with several pond members very close to the road (Table 2, Table 3). Clusters c2 - c5 are all significantly affected by road avoidance; in contrast to c6 in which the majority of member ponds are further away from the road (Table 2, Table 3).
Effect of road avoidance (Ra) and road mortality (Rd) on within-cluster connectivity (Sc) in cluster c2 – c6.
As hypothesized road mortality has a negative effect on between-cluster connectivity. However, contrary to our expectation we find that road avoidance can promote connectivity across roads. An explanation could be that roads actually functions as traps if road avoidance is low. In this model roads are not just lines to cross; they are considered the same way as other kinds of habitat. Hence, when habitat attraction of the roads is higher (and thus road avoidance low) than the surrounding habitat, the road may actually be the preferred habitat. Moreover, the survival probability on roads is always lower than in any other type of habitat. Thus, at high levels of road avoidance, frog agents only rarely enter the roads, but if they do they quickly leave it again and only suffer the high mortality for at short time. When road avoidance is low, frog agents enter the roads more willingly and will tend to stay there, suffering from the higher mortality for a longer time. The severity of the “trap” effect will depend on road mortality as, all else being equal, successful dispersal across the road depends on the survival probability. The results do in fact show a strong interaction; the positive effect of road avoidance on between-cluster connectivity getting more pronounced as road mortality increases.
In accordance with our second hypothesis, we find that road avoidance has a positive effect on local connectivity. In particular, when road avoidance is set to five, connectivity shows considerable elevated values. The strong effect on within-cluster connectivity of a 100 % barrier may seem surprising, but can be explained as a “deflection” effect. When the road is inaccessible road mortality is no longer an issue and a larger proportion of frog agents will survive. Moreover, the blockage forces the agents to move along the road instead of crossing. Taken together, this has the effect that a larger proportion of frog agents stays within the local area for a longer time, which increases the probability of an agent settling within the cluster, enhancing within-cluster connectivity. We did not expect road mortality to have an effect on within-cluster connectivity, but we do find a negative, although week, response. This is probably because there will be a small proportion of frog agents entering the road and returning to the same side. The survival probability of these returnees will depend on road mortality.
The seven clusters identified in this study do not all respond in the same way to changes in road avoidance and road mortality. Two of the clusters, c1 and c7, are not affected at all; these are also the clusters furthest away from the road. Road mortality only significantly affects larger clusters with several pond members very close to the road; maybe because only these clusters have sufficient number of returnees for the effect to be detectable. Road avoidance, on the other hand, affects also clusters further away; only clusters with a minimum distance to road above 300 m are unaffected by road avoidance. The result suggests that if the road is within the summer habitat of some of the member ponds, then road avoidance will affect within-cluster connectivity.
In this study, scenarios with road avoidance set to five, correspond to real life situations where fencing along roads prevents access to the road. Our results suggest that fencing can result in highly increased local connectivity, even between ponds not in immediate proximity to the road. Thus, fencing may not just mitigate road induced mortality but may actually enhance local population persistence. However, the results also show this to be at the cost of regional connectivity. Fencing separates a population into several smaller and more isolated groups of subpopulations, each of which may have a higher risk of extinction and a lower probability of recolonisation. In a simulation experiment with a local population of virtual animals
The series of scenarios are hypothetical and all may not correspond to real life situations but road mortality can indeed range between very low and very high values, depending on traffic intensity. Extreme low values of road avoidance, to the point where the road becomes more attractive than the surrounding landscape, may seem very unrealistic. However, behavioural responses to traffic like immobilisation (
Our study concerns a specific landscape and a specific species, but still it is possible to draw some general conclusion. First of all our results emphasize that connectivity is context dependent. The behaviour of the focal species, the structure of the landscape and their interaction are essential to how connectivity is realized. Furthermore, our simulations indicate that the barrier effect of roads not only affect dispersal across roads. Even between ponds located on the same side of the road, dispersal success can be highly susceptible to road avoidance and road mortality, depending on the distance to the road. This suggests that roads may affect not only regional or metapopulation dynamics but also have a direct effect on local population dynamics.
Very little is known about the effects of mitigation measures in general. Once mitigation measures are implemented, efforts are seldom put into discovering how well they work (
The work was funded by the Danish Road Directorate. The Amphi Consult group has provided amphibian expertise as well as map data. Marianne Ujvári, Martin Hesselsøe, Agnete Jørgensen and Martin Schneekloth have given valuable feed-back during the model development. Uta Berger has given precious help during the work and kept the main author on the IBM-track. We are very thankful to Carolyn Bauer for linguistic help. We also thank two anonymous reviewers for insightful comments on the manuscript.
Full model description following the ODD-template suggested by