Citation: Oppel S, Hervías S, Oliveira N, Pipa T, Silva C, Geraldes P, Goh M, Immler E, McKown M (2014) Estimating population size of a nocturnal burrow-nesting seabird using acoustic monitoring and habitat mapping. Nature Conservation 7: 1–13. doi: 10.3897/natureconservation.7.6890
Population size assessments for nocturnal burrow-nesting seabirds are logistically challenging because these species are active in colonies only during darkness and often nest on remote islands where manual inspections of breeding burrows are not feasible. Many seabird species are highly vocal, and recent technological innovations now make it possible to record and quantify vocal activity in seabird colonies. Here we test the hypothesis that remotely recorded vocal activity in Cory’s shearwater (Calonectris borealis) breeding colonies in the North Atlantic increases with nest density, and combined this relationship with cliff habitat mapping to estimate the population size of Cory’s shearwaters on the island of Corvo (Azores). We deployed acoustic recording devices in 9 Cory’s shearwater colonies of known size to establish a relationship between vocal activity and local nest density (slope = 1.07, R2 = 0.86, p < 0.001). We used this relationship to predict the nest density in various cliff habitat types and produced a habitat map of breeding cliffs to extrapolate nest density around the island of Corvo. The mean predicted nest density on Corvo ranged from 6.6 (2.1–16.2) to 27.8 (19.5–36.4) nests/ha. Extrapolation of habitat-specific nest densities across the cliff area of Corvo resulted in an estimate of 6326 Cory’s shearwater nests (95% confidence interval: 3735–10, 524). This population size estimate is similar to previous assessments, but is too imprecise to detect moderate changes in population size over time. While estimating absolute population size from acoustic recordings may not be sufficiently precise, the strong positive relationship that we found between local nest density and recorded calling rate indicates that passive acoustic monitoring may be useful to document relative changes in seabird populations over time.
Cory’s shearwater, Calonectris borealis, vocal activity, nest density, Random Forest, Azores, Macaronesia, Procellariformes
Seabirds are globally the most threatened group of birds (
Monitoring the population size of nocturnal burrow-nesting seabirds has recently benefited from autonomous acoustic recording devices, which can be deployed on remote islands to record the vocal activity of seabirds (
Here we present a case study for estimating the population size of Cory’s shearwater (Calonectris borealis, recently split from Calonectris diomedea (
Corvo is a small (1700 ha) island of volcanic origin located in the central North Atlantic (39°40'N, 31°7'W). The volcanic cone of the island rises to 718m, and due to wind and wave action much of the volcanic cone has eroded, particularly on the western coast. The erosion has led to almost vertical cliffs between 200–600 m tall along the majority (16.3 km) of Corvo’s coastline. Due to the inaccessible nature of the cliffs, the size of the Cory’s shearwater population has never been quantified (
In May 2011 and 2012, we deployed a total of nine autonomous acoustic recorders (SongMeter SM2, Wildlife Acoustics Inc., Concord, MA) in colonies that were expected to have varying nest density of Cory’s shearwaters, but where all burrows and potential nest cavities within a 50 m radius could be manually inspected to assess local nest density. These colonies were situated on Corvo as well as on the islands of Faial (38°35'N, 28°48'W), and Vila Franca do Campo (37°42'N, 25°26'W) in habitats similar to the cliffs on Corvo. In May 2012, we deployed 12 additional acoustic recorders in various cliff habitats on Corvo where nest density assessment was not possible, including near-vertical cliffs where recorders were deployed with ropes. All recorders were deployed in wind-sheltered areas on the ground or a cliff ledge, with two independent microphones elevated 30 cm above ground and spaced < 50 cm apart. Recorders operated on an identical schedule for the entire breeding season (late May to mid October), with 1 min recordings every 10 min from local sunset to local sunrise. Gain on both independent microphones was set to the default of +42.0 dB and sound was recorded at a sample rate of 16 kHz in stereo.
In June 2011 and 2012, we searched for occupied shearwater burrows within a 50 m radius of the nine accessible recorders, based on the assumption that SongMeters can record vocalisations up to 50 m away (
Vocal activity of burrow-nesting seabirds at colonies is dependent on many environmental factors and thus varies considerably within nights and over the breeding season (
Due to the long deployment period, the recorded vocal activity could not be assessed manually but required an automated call recognition algorithm (
To calibrate the relationship between local nest density and vocal activity, we used the nine accessible recorders where local nest density was known. Because we expected vocalisations to increase linearly with nest density, we fitted a linear regression to the mean number of shearwater calls per minute with nest density as dependent variable. This linear relationship was then used to predict local nest density at the remaining 12 recorders that were placed in locations where nest burrows could not be manually surveyed.
To be able to extrapolate local nest density assessed via acoustic recorders to the entire suitable nesting area for Cory’s shearwaters on Corvo, we adopted a habitat modelling approach to predict nest density in different cliff micro-habitats following similar work in mountainous areas (
The composite cliff panoramas were visually inspected and homogenous areas of similar habitat type were manually delineated as polygon features in a geographic information system (ArcMap 10.1, ESRI Inc., Redlands, CA). Each delineated polygon was given a value for three habitat features (
The same three habitat features were also recorded around the location of each acoustic recording device. This allowed us to use the estimated nest density inferred from the recorded calling rate in a habitat model to predict the nest density in relation to the three habitat features, and thus assess density in those combinations of habitat features where no recorder had been placed.
We first estimated the local nest density for all recording units based on the acoustic calibration relationship described above. We then related the estimated nest density at each recorder to the three habitat features to establish a predictive relationship between the level of each habitat feature and nest density (
To extrapolate from nest density to total population size of Cory’s shearwaters, we used the habitat feature map derived from digital photographs to calculate the proportion of the entire cliff area that was covered by polygons with each combination of habitat features. The proportion of each habitat type was multiplied by the entire area of suitable cliff habitat around Corvo, estimated from the length of the coastline (16.3 km) and the height of cliffs to be 490 ha.
We then summed the number of shearwater nests predicted to occur in each habitat across the entire island to derive an estimate of island-wide breeding population size of Cory’s shearwaters. We present the estimate of breeding population size with 95% confidence intervals derived from the linear regression predicting nest density around each recorder.
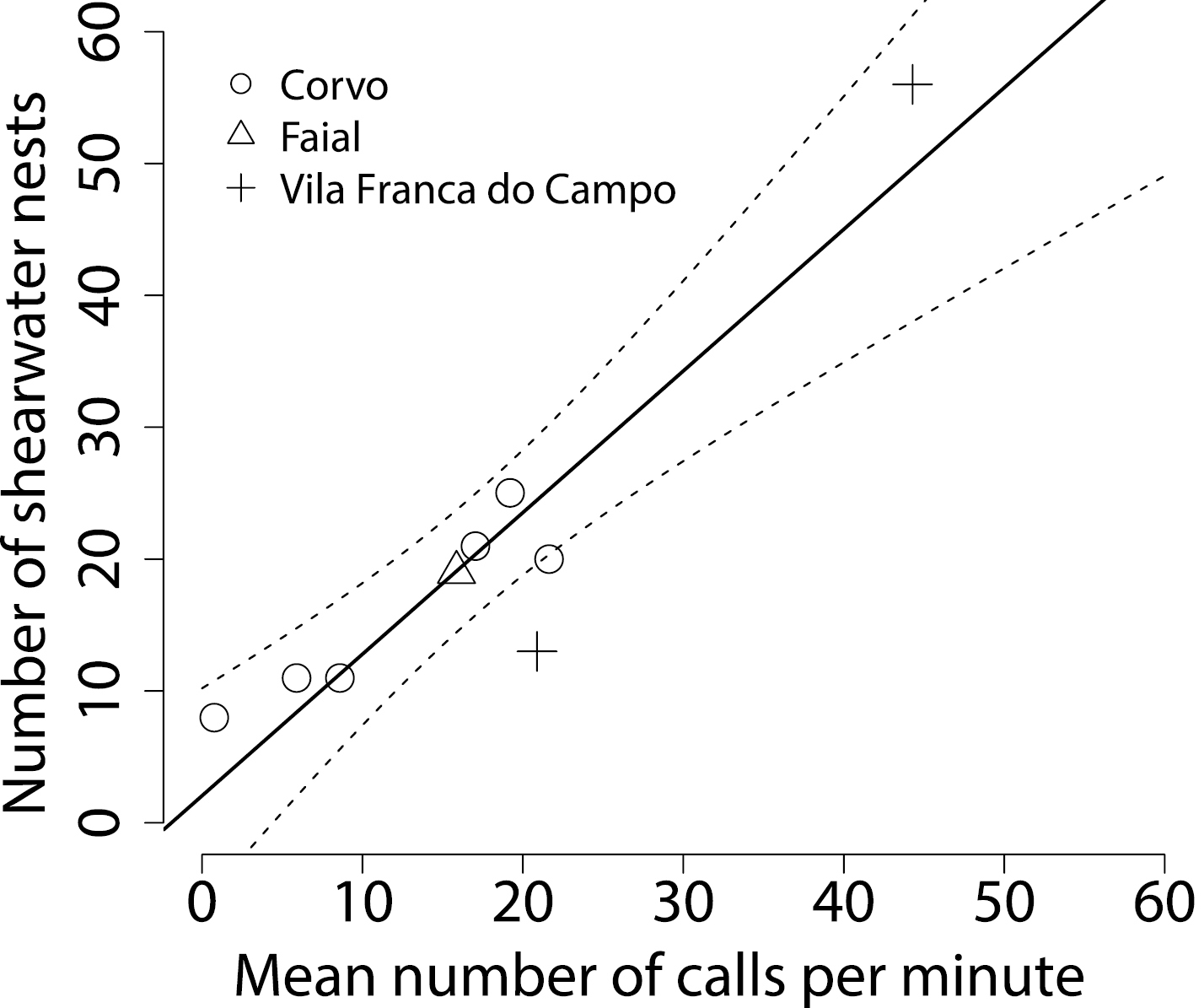
The mean calling rate per 1-min recording ranged from 0.7 to 55.2 Cory’s shearwater calls at the nine recorders with known local nest density, and from 0–25.1 calls at the recorders placed at inaccessible cliff locations. We counted between 8–56 occupied Cory’s shearwater burrows in a 50 m radius around accessible recorders, and found a relationship that indicated a linear increase in local nest density with increasing calling rate (slope = 1.07, R2 = 0.86, p < 0.001; Fig. 1). Based on this relationship, the mean predicted nest density around the 12 recorders where no nest count had been feasible was 9.5 nests/ha (95% confidence interval 4.1–18.5 nests/ha).
The number of occupied Cory’s shearwater nests within a 50 m radius around acoustic recording devices increased with mean shearwater call rates measured during the 2011 and 2012 Cory’s shearwater breeding seasons (solid line = linear regression with slope = 1.07, R2 = 0.86, p < 0.001; broken line = 95% confidence intervals). Different symbols represent data from three islands in the Azores, North Atlantic Ocean.
The 21 acoustic recorders were placed in nine different combinations of the three habitat variables and represented all levels of the three habitat features. The Random Forest habitat model relating estimated nest density to habitat features performed well in cross-validation and observed and predicted nest densities were positively correlated (Pearson r = 0.73, p < 0.001). This model predicted that nest density across all combinations of habitat features on Corvo ranged from 6.6 (2.1–16.2) to 27.8 (19.5–36.4) nests/ha (Table 1).
Highest nest densities were predicted either on vertical cliffs (> 85° inclination) with flat rock, or in less steep areas (< 60°) with deep soil suitable for excavating burrows. Lowest nest densities were predicted in areas with intermediate inclination (60-85°) and no flat rock (Table 1). Extrapolating the estimated habitat-specific densities across the entire cliff area of Corvo resulted in an estimate of 6326 Cory’s shearwater nests (95% confidence interval: 3735–10, 524).
The distribution of estimated nest densities and number of estimated Cory’s shearwater nests (with 95% confidence intervals) around the 490 ha of cliff habitat on the island of Corvo (Azores) in 2012.
| Soil Type | Rock type | Inclination | Area (ha) | Nest density (nests/ha) | N nests |
|---|---|---|---|---|---|
| deep | flat rock | <60° | 2.2 | 27.8 (19.5–36.4) | 62 (43–81) |
| deep | flat rock | >85° | 3.8 | 23.5 (15.9–31.9) | 89 (60–120) |
| deep | broken rock | <60° | 45.1 | 23.3 (17.2–30.3) | 1050 (776–1367) |
| shallow | flat rock | >85° | 3.9 | 23.1 (15.9–31.2) | 90 (62–121) |
| shallow | flat rock | <60° | 1.8 | 22.1 (14.8–31) | 40 (27–56) |
| deep | flat rock | 60-85° | 2.3 | 17.1 (10.4–26.6) | 40 (24–62) |
| deep | broken rock | >85° | 82.4 | 16.7 (11.2–24.4) | 1378 (923–2012) |
| deep | no rock | <60° | 3.3 | 16.3 (9.2–24.7) | 54 (30–81) |
| shallow | flat rock | 60-85° | 0.2 | 15.5 (9.4–24.9) | 4 (2–6) |
| shallow | broken rock | >85° | 5.3 | 13.8 (8–22) | 72 (42–116) |
| shallow | broken rock | <60° | 71.2 | 13.4 (7.9–22) | 955 (565–1565) |
| deep | no rock | >85° | 1.7 | 12.5 (6.3–20.9) | 22 (11–37) |
| shallow | no rock | <60° | 89.8 | 11.1 (5.2–20.1) | 1001 (466–1808) |
| deep | broken rock | 60–85° | 92.5 | 10.3 (5.8–19.3) | 949 (541–1785) |
| deep | no rock | 60–85° | 2.6 | 8.2 (3–17.4) | 21 (8–45) |
| shallow | no rock | 60–85° | 35.8 | 6.7 (1.9–16.2) | 241 (68–581) |
| shallow | broken rock | 60–85° | 42.6 | 6.6 (2.1–16.2) | 283 (88–691) |
Based on acoustic recording and habitat mapping we estimated that >6000 pairs of Cory’s shearwaters nested on Corvo in 2012. This population size estimate is surrounded by considerable uncertainty (3735–10, 524 pairs), which describes the potential range of the Cory’s shearwater population on Corvo. Due to this large uncertainty our estimate is unlikely to serve as a useful baseline for assessing moderate changes in population size.
Our population size estimate is of a similar magnitude as previous extrapolations for Corvo (6000–12, 000 pairs in 1996), which were derived from counting individuals rafting at sea or multiplying average breeding densities by the area of available habitat (
The large uncertainty in our abundance estimates is a consequence of error propagation across two different model predictions – the predicted nest density based on recorded calling rate, and the predicted overall abundance extrapolated from the predicted nest density per habitat type. Additional uncertainty may arise because nest density may vary due to social attraction and the presence of invasive predators in addition to suitable habitat (
Despite the imprecise population size estimate, our work suggests that there is a positive relationship between calling rate recorded by autonomous acoustic recorders and seabird nest density. This finding builds on previous work (
This work was co-financed by the European Union (LIFE07 NAT/P/000649).We thank A. Díez, J. Bried, J. Katzenberger, J. Landschoff, J. Roma, J. Benedicto, J. Garcia, J. Teodósio, K. Cunningham, K. Puttick and S. Monforte for help in fieldwork and P. Domingos for his friendly and material support. We appreciate the support and advice of R. Buxton, A. Borker, N. Boucher, S. Snyder and J. Murray on acoustic data processing. S. Boggio and several volunteers digitised the cliff habitat map from photos and their contribution was invaluable. K. Camphuysen and two anonymous reviewers provided helpful comments on an earlier draft of this manuscript.